Abstract
Background: Older patients (>60 years) with acute myeloid leukemia (AML) face difficult treatment decisions as they can be treated either with risky multi-drug "intensive" chemotherapy for a small chance of a cure, or "non-intensive" and non-curative palliative chemotherapy. To make informed decisions about their treatment, patients must have a good understanding of their prognosis and overall risks of these treatment options. However, studies examining patients' understanding of their prognosis and treatment risk are lacking.
Methods: We conducted a longitudinal study of older patients newly diagnosed with AML at two tertiary care hospitals. At enrollment, we assessed patients' and oncologists' perception of treatment-related mortality. At one month, we assessed patients' and oncologists' perception of prognosis using the Prognosis and Treatment Perception Questionnaire. We also examined whether patients discussed their end-of-life care wishes with their oncologist at 24 weeks. We generated descriptive statistics for all variables and stratified one-month patient data by chemotherapy type (intensive versus non-intensive). We compared patients' perceptions of prognosis and treatment-related mortality with their oncologists' perceptions using chi-square tests.
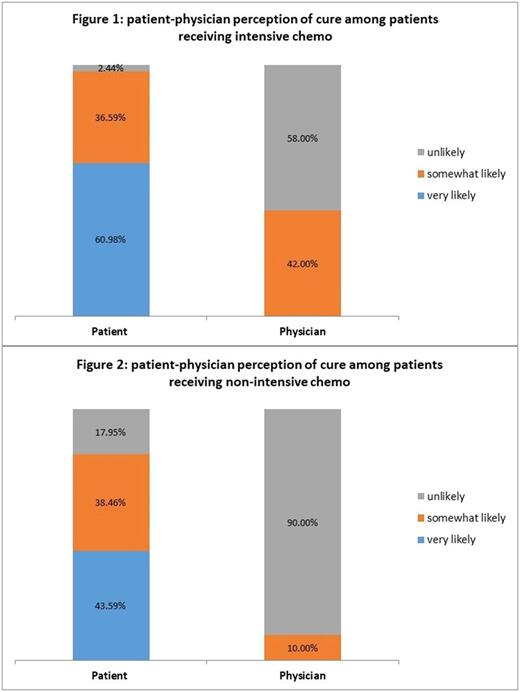
Results: We enrolled consecutive patients within 72 hours of initiating intensive (n = 50) or non-intensive (n = 50) chemotherapy. The majority of patients reported that it is at least 'somewhat' likely to die due to treatment (91.3%, 84/92) while their oncologists reported that it is 'very unlikely' (80.0%; 74/92) for the patient to die due to their treatment (P < 0.001). Although most patients (95.06%, 77/88) reported it was 'very important' to know their prognosis, there were substantial discrepancies in their prognostic understanding. Most patients (90.0%, 73/81) reported that they were 'somewhat' or 'very likely' to be cured of their leukemia while most oncologists reported that it is 'unlikely' or 'very unlikely' for the patient to be cured (74% (60/81)) (P < 0.001). Patients receiving both intensive and non-intensive chemotherapy had significant misperceptions about their prognosis. Among patients receiving intensive chemotherapy, 97.6% reported that they are 'somewhat' or 'very likely' to be cured, while 58% of their oncologists reported that cure was unlikely (P < 0.001) [Figure 1]. Among patients receiving non-intensive chemotherapy, 82.1% reported that they are 'somewhat' or 'very likely' to be cured, while 90% of their oncologists reported that cure was unlikely (P < 0.001) [Figure 2]. Most patients (77.8%, 42/54) have not discussed their end-of-life care wishes with their oncologists 24 weeks after initiating therapy for AML.
Conclusion: Older patients with AML have substantial misperceptions regarding the risks of their treatment and they overestimate the likelihood of cure, compared to their oncologists' estimates. Prognostic misperceptions are especially striking in patients receiving non-intensive chemotherapy. Despite the oncologists' assessment that their prognosis was poor, most older patients with AML did not report discussing their end-of-life care preferences with their oncologists 24 weeks after initiating therapy for their disease. These data underscore the critical gaps in communication and prognostic understanding in order patients with AML. Interventions are needed to improve communication with oncologists and to ensure older patients with AML have an accurate understanding of their treatment risks and prognosis to make informed decisions about their treatment.
Fathi: Pfizer: Honoraria; Takeda: Research Funding; Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Steensma: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; H3 Biosciences: Consultancy; Onconova: Consultancy; Incyte: Equity Ownership; Janssen: Consultancy, Research Funding; Celgene: Consultancy; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy. LeBlanc: Helsinn Therapeutics: Consultancy, Honoraria; Janssen: Honoraria; Flatiron Health: Consultancy; AstraZeneca: Research Funding; Celgene: Honoraria; American Cancer Society: Research Funding; Seattle Genetics: Research Funding; Pfizer: Consultancy; Otsuka: Membership on an entity's Board of Directors or advisory committees; Cambia Health Foundation: Research Funding; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees. DeAngelo: Glycomimetics: Research Funding; Incyte: Consultancy, Honoraria; Celgene: Research Funding; Blueprint Medicines: Honoraria, Research Funding; ARIAD: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; Shire: Honoraria; Takeda Pharmaceuticals U.S.A., Inc.: Honoraria; BMS: Consultancy; Immunogen: Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Pfizer Inc.: Consultancy, Honoraria, Research Funding. Stone: Novartis: Consultancy; Arog: Consultancy; Pfizer: Consultancy; Ono: Consultancy; Sumitomo: Consultancy; Celgene: Consultancy; Fuji Film: Consultancy; Agios: Consultancy; Amgen: Consultancy; Astellas: Consultancy; Jazz: Consultancy; Abbvie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal